This blog dives deep into the heart of primary legal authority with a rigorous analysis of key 2nd Circuit decisions related to food and consumer-product litigation.
Allegedly ineffective "charcoal" in Colgate Toothpaste ruled not false or misleading

2023: The "charcoal" marketed in Colgate's Optic White toothpaste is not harmonized with other claims like "enamel safe" and "whole mouth health," according to the class-action plaintiffs. Instead, "[t]he consensus of respected dentists, researchers, and industry experts weighs against the use of charcoal toothpaste because they are not safe to use, contrary to Defendant's Safety Claims."
But, the court ruled the plaintiff's evidence did not prove that charcoal in the toothpaste undermined all enamel benefits. The studies did not involve "the actual Colgate toothpaste at issue." Even worse, two other complaints that relied on the same studies were dismissed. The case is Bermudez v. Colgate-Palmolive Co., 1:21-CV-10988 (JLR), 2023 WL 2751044, at *11 (S.D.N.Y. Mar. 31, 2023). Here is the complaint.
At best, the studies raised an "inference" that charcoal may harm tooth enamel. Nor do the plaintiffs allege that Colgate's "toothpaste contains charcoal that is sufficient to render the toothpaste unsafe and incapable of providing the advertised benefits."
In exercising legal authority to determine the plausibility of allegations, courts "regularly look to documents referenced in a complaint and make such determinations on a motion to dismiss. Considering unsupportive studies to hold against plaintiffs is an acceptable practice. Two cases to support this finding:
- Segovia v. Vitamin Shoppe, Inc.: Studies referenced in the complaint that do not support the legal theories may be defective. It is "well settled that a court may make this determination as a matter of law." No. 14-cv-07061 (N.S.R.), 2016 WL 8650462, at *8 (S.D.N.Y. Feb. 5, 2016).
- Kardovich, 97 F. Supp. 3d at 140-41: If studies do not support the complaint, a court may dismiss them "without considering "issues of fact, credibility, and the weight of the evidence."
Generalized scientific studies that do not directly support a theory do "not plausibly allege a false, misleading, or deceptive act," held the court. Regarding the plaintiff's fraud claim, the complaint was "insufficient to constitute strong circumstantial evidence of conscious misbehavior or recklessness." The case was dismissed.
Claims against TRESemmé Shampoo for causing dermatitis survive dismissal
2023: The class-action plaintiff suffered an “increasingly severe reaction” to TRESemmé shampoo with “significant hair loss, hair thinning, and severe scalp irritation,” according to her complaint.

![]()
The shampoo contained “DMDM, a hydantoin compound used in cosmetics as a preservative.” “DMDM acts as a ‘formaldehyde donor’ that slowly releases formaldehyde in order to prevent microbial growth and extend the shelf life of cosmetics...” read the complaint.
The FDA classifies DMDM as a common allergen known to cause “itchiness, red rashes on the skin, or more extreme reactions.,” alleged the plaintiff. Using the shampoo may induce “irritant contact dermatitis” when DMDM causes “direct epidermal keratinocyte damage,” alleged the plaintiff. Competing shampoo providers have allegedly avoided using such “potentially harmful chemicals.”
When the plaintiff discontinued using TRESemmé shampoo, her dermatitis symptoms stopped, thus implicating the shampoo as a causal factor of her dermatitis.
Defendant Unilever argued that other factors, such as “alopecia, stress, diet, or medications,” may have caused the plaintiff’s hair loss. But these “potential alternatives” do not make the plaintiff’s DMDM theory implausible, held the court. Nor was the plaintiff required to prove that DMDM was an allergen. Unilever cited a case, Dunham, which established a lack of causation due to a more likely alternate cause. Still, such an alternative is not apparent in this case.
To the extent the plaintiff pleads an allergy, she pointed to the FDA's classification of DMDM "as one of the top allergens that cause the most allergic reactions from the use of cosmetic products.'" The fact that Unilever disclosed DMDM in its ingredients did not preclude the plaintiff's failure-to-warn theory.
Additional legal authorities cited in Candelaria v. Conopco, Inc.:
- "In a claim for defective design, plaintiff must make a showing that (1) the product as designed posed a substantial likelihood of harm; (2) it was feasible to design the product in a safer manner; and (3) the defective design was a substantial factor in causing plaintiff's injury." Colon ex rel. Molina v. BIC USA, Inc., 199 F. Supp. 2d 53, 83 (SDNY 2001) (citing Voss v. Black & Decker Mfrg. Co., 59 N.Y.2d 102, 108 (1983)).
- Under New York Law, failure-to-warn claims require pleading "(1) that a manufacturer has a duty to warn; (2) against dangers resulting from foreseeable uses about which it knew or should have known; and (3) that failure to do so was the proximate cause of harm." Colon ex rel. Molina, 199 F. Supp. 2d at 84.
- "The adequacy of the warning in a products liability case based on a failure to warn is, in all but the most unusual circumstances, a question of fact to be determined at trial." Vicuna v. O.P. Schuman & Sons, Inc., 298 F. Supp. 3d 419, 439 (EDNY 2017) (quoting Cooley v. Carter–Wallace Inc., 102 A.D.2d 642, 642 (N.Y. App. Div. 4th Dep't)).
10 Takeaways from Candelaria v. Conopco, Inc.:
- To plead causation, a plaintiff need not eliminate every other potential cause of their injury. They only need to plead facts that plausibly show the defendant's actions or product to be a substantial factor in their injury.
- A defendant's speculation about other possible causes of a plaintiff's injury does not make it implausible that the defendant's product was a substantial factor in the injury.
- The fact that a plaintiff is not allergic or hypersensitive to a product's ingredient does not render causation implausible, if the complaint doesn't allege that the ingredient only causes harm in allergic individuals.
- In a claim for defective design, a plaintiff must demonstrate that the product as designed posed a substantial likelihood of harm, it was feasible to design the product in a safer manner, and the defective design was a substantial factor in causing plaintiff's injury.
- An injury based on an allergy is not a valid basis for finding a product defect.
- A failure-to-warn claim under New York law requires pleading that a manufacturer has a duty to warn against foreseeable dangers it knew or should have known about, and failure to do so was the proximate cause of harm.
- The adequacy of warning in a products liability case based on a failure to warn is usually a question of fact to be determined at trial.
- A plaintiff can fulfill the requirement to plead the commonality of an allergy for a failure-to-warn claim by including a recognized authority's classification of the allergen.
- A plaintiff's inability to satisfy Rule 23(a)(1)'s numerosity requirement does not justify striking class allegations at the pleading stage.
- A motion to strike class allegations before class certification can be granted when it raises issues separate from those to be decided on a class certification motion, or when it demonstrates that certification will be impossible regardless of facts obtained during discovery.
Dove's "100% Natural," "microbiome gentle" bodywash ruled plausibly misleading given synthetic ingredients
2022: Dove's "microbiome gentle" bodywash was allegedly false because it "contains ingredients (oils, preservatives, surfactants, etc.) which trigger negative skin reactions" and disrupts the "microbiome," alleged the plaintiff.

Aside from the false promise of microbiome benefits, reasonable consumers would not expect synthetic ingredients in the "100% Natural" bodywash. The case is Anderson v. Unilever U.S., Inc., 607 F. Supp. 3d 441 (S.D.N.Y. 2022).
Some allegedly toxic ingredients in the Dove Bodywash:
- Coccamidopropyl betaine ("CAPB" ): "unsafe when not thoroughly rinsed off the skin"
- Sodium lauroyl glycinate: "known to be disruptive to the microbiome because of its pH level"
The allegedly deceptive advertising claims:
- "Our moisturizing and microbiome gentle formula provides instant softness and lasting care for your skin."
- "deep moisture"
- "skin-natural nourishers"
- "instantly soft skin, lasting nourishment"
- "moisture renew blend"; and "nourishing body wash."
- A graphic with the phrase "microbiome gentle" next to an icon of a double helix.
- "Start your year with a healthy microbiome."
- "Your microbiome is a protective layer that helps keep skin healthy, moisturized[,] and resilient. Wash gently and revitalize skin with microbiome-gentle Dove."
- "What you eat isn't the only thing that may affect your skin's microbiome—skin's living protective layer."
The plaintiff alleged enough facts to survive a motion to dismiss. Other cases with similar fact patterns include:
- Paulino v. Conopco, Inc.: Plaintiffs sufficiently alleged that 'Naturals' labeling misled them since they were "filled with unnatural, synthetic ingredients." No. 14-CV-5145, 2015 WL 4895234, at *6 (E.D.N.Y. Aug. 17, 2015).
- Goldemberg v. Johnson & Johnson Consumer Cos.: "Active Naturals" claim in a product that contained synthetic ingredients survived a motion to dismiss. 8 F. Supp. 3d 467, 478–80 (S.D.N.Y. 2014).

The "relevant question is not whether Defendant's representations are, formally speaking, correct, but whether a reasonable consumer might be misled." See Twohig, 519 F. Supp. 3d at 160. Anderson v. Unilever U.S., Inc., 607 F. Supp. 3d 441 (S.D.N.Y. 2022).
Whether "microbiome gentle" refers to the bodywash's formula or its ingredients was not ripe for a court decision. "A reasonable consumer could interpret Defendant's representations in the manner that Plaintiff alleges," held the court. The ingredient list did not cure the misimpression.
Lastly, the plaintiff's "price premium" theory—overpayment based on misrepresentation—was enough to state a claim for relief. But the allegations did not support the intent to defraud (scienter) necessary for a fraud claim.
Additional legal authorities cited in Anderson v. Unilever U.S., Inc.:
- A company's general profit motive is insufficient to plead scienter for a fraud claim. Fed. R. Civ. P. 9(b). Anderson v. Unilever U.S., Inc. at 441.
- "A defendant engages in 'consumer-oriented' activity if [the company's] actions cause any 'consumer injury or harm to the public interest.'" New York v. Feldman, 210 F. Supp. 2d 294, 301 (S.D.N.Y. 2002) (quoting Securitron Magnalock Corp. v. Schnabolk, 65 F.3d 256, 264 (2d Cir. 1995)). "This requirement is liberally construed, id., and "may be satisfied by showing that the conduct at issue 'potentially affect[s] similarly situated consumers,'" Wilson v. Nw. Mut. Ins. Co., 625 F.3d 54, 64 (2d Cir. 2010) (alteration in original) (quoting Oswego Laborers' Local 214 Pension Fund v. Marine Midland Bank, N.A., 85 N.Y.2d 20, 623 N.Y.S.2d 529, 647 N.E.2d 741, 745 (1995)). Anderson v. Unilever U.S., Inc. at 451 (S.D.N.Y. 2022).
- Legal theories against advertising claims are defective as a matter of law when ad interpretations are 'patently implausible' or 'unrealistic." Eidelman v. Sun Prods. Corp., No. 16-CV-3914, 2017 WL 4277187, at *4 (S.D.N.Y. Sept. 25, 2017) (quoting Stoltz v. Fage Dairy Processing Indus., S.A., No. 14-CV-3826, 2015 WL 5579872, at *20 (E.D.N.Y. Sept. 22, 2015)); see also In re Frito-Lay N. Am. Inc. All Nat. Litig., No. 12-MD-2413, 2013 WL 4647512, at *16 (E.D.N.Y. Aug. 29, 2013) (explaining that claims which "border on fantasy" require "dismissal as a matter of law"). Anderson v. Unilever U.S., Inc. at 452 (S.D.N.Y. 2022).
- "Where a representation is capable of two possible reasonable interpretations," the Court is not free to reject "the misleading one ... simply because there is an alternative, non-misleading interpretation." Fishon v. Peloton Interactive, Inc., No. 19-CV-11711, 2020 WL 6564755, at *7 (S.D.N.Y. Nov. 9, 2020).
Rite Aid's infant & children fever reducer ruled not materially misleading given the labels' "prominent and accurate disclosures"
2021: A mother claimed she was duped into paying more for an "Infant" fever reducer when the lower-cost "Children" version contains the same amount of acetaminophen, the active ingredient.
Here is the class-action complaint. The case is Ostermeier-McLucas v. Rite Aid Hdqtrs. Corp., 549 F. Supp. 3d 276 (E.D.N.Y. 2021).
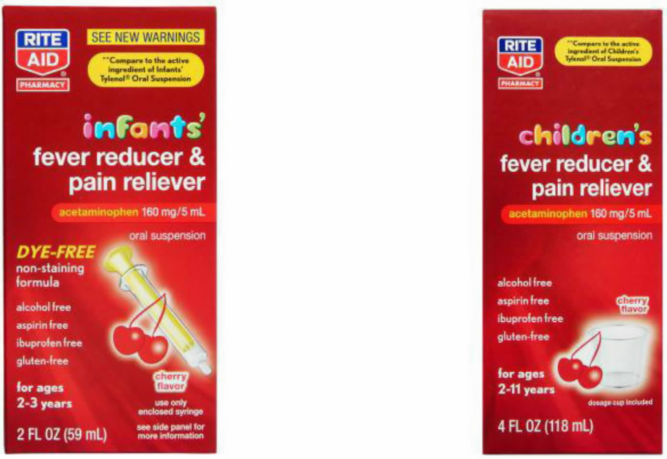
Rite Aid's fought back, arguing that no reasonable consumer would have been misled because the ingredients could be easily seen. The court sided with the defendant stating the plaintiff has not shown that "a significant portion of the general consuming public or targeted customers, acting reasonably in the circumstances, could be misled by Rite Aid's labels."
The court noted two recent California decisions dismissing analogous cases: Lokey v. CVS Pharmacy, Inc. and Eidmann v. Walgreen Co. Both involved infant acetaminophen, and both packages accurately disclosed their respective amounts. Here, the Rite Aid plaintiff's claims failed "as a matter of law" for the same reasons.
Additional legal authorities cited in Ostermeier-McLucas v. Rite Aid Hdqtrs. Corp.:
- "To state a claim, 'plaintiffs must do more than plausibly allege that a 'label might conceivably be misunderstood by some few consumers.'"Jessani v. Monini N. Am., Inc., 744 F. App'x 18, 19 (2d Cir. 2018).
- Instead, they must "plausibly allege 'that a significant *283 portion of the general consuming public or of targeted consumers, acting reasonably in the circumstances, could be misled.'" Id. (quoting Ebner, 838 F.3d at 965); see also Fermin v. Pfizer Inc., 215 F. Supp. 3d 209, 211 (E.D.N.Y. 2016) ("The term 'likely' indicates that deception must be probable, not just possible."). This standard is an objective one. Ostermeier-McLucas v. Rite Aid Hdqtrs. Corp., 549 F. Supp. 3d 276, 282–83 (E.D.N.Y. 2021).
- No deception claim is permitted when the "allegedly deceptive practice was fully disclosed" in the advertisements. Chufen Chen v. Dunkin’ Brands, Inc., 954 F.3d 492, 501 (2d Cir. 2020).
- Different sizing of ibuprofen packaging was ruled not misleading because "plaintiffs provided no basis for disregarding the clearly stated pill-counts on the labels." Fermin, 215 F. Supp. 3d at 212.
- A "reasonable consumer would not be misled where the alleged deceptions "are eclipsed by the accurate disclosure statement" on the packaging. Bowring v. Sapporo U.S.A., Inc., 234 F. Supp. 3d 386, 391 (E.D.N.Y. 2017). Ostermeier-McLucas v. Rite Aid Hdqtrs. Corp., 549 F. Supp. 3d 276, 284 (E.D.N.Y. 2021).

Dunkin' Doughnuts' "Angus Steak" advertisements were not materially misleading
2020: A class-action plaintiff alleged that Dunkin' Doughnuts deceptively marketed two of its sandwiches:
- Angus Steak & Egg Breakfast Sandwich
- Angus Steak & Egg Wake-Up Wrap

Specifically, the plaintiff alleged that Dunkin' Doughnuts, through its labeling and television advertisements, deceptively implied that they'd receive an "intact piece of meat" instead of "ground beef patty with multiple additives." The case is Chufen Chen v. Dunkin' Brands, Inc., 954 F.3d 492, 495 (2d Cir. 2020). Here is the class-action complaint.
The lower court found that:
- "Angus Steak" was not deceptive or misleading to a reasonable consumer;
- "Angus Steak" was not a warranty under the federal Magnuson-Moss Act;
- New York was the proper venue for out-of-state plaintiffs since Dunkin' Doughnuts was not subject to "general jurisdiction" merely by being registered here under Business Corporation Law § 1301. Dunkin' Doughnuts is incorporated in Delaware and headquartered in Massachusetts.
This court affirmed the dismissal.
The definition of "Steak" also includes "ground beef," resembling the pictures
The pictures clearly display the "steak" as a "patty." "While the word 'steak' can refer to 'a slice of meat,' it is also defined as 'ground beef prepared for cooking or for serving in the manner of a steak.' Merriam-Webster Online Dictionary. "Classic examples of ground beef served as 'steak' include chopped steak, hamburger steak, and Salisbury steak." Chufen Chen v. Dunkin' Brands, Inc., 954 F.3d 492, 501 (2d Cir. 2020).
When courts analyze misleadingness, "context is crucial." Here, the plaintiff "bought her Angus Sandwich for less than $4 and her Angus Wrap for less than $2." The product is marketed as "grab-and-go," and reasonable consumers would not be "misled into thinking she was purchasing an "unadulterated piece of meat." Chufen Chen v. Dunkin' Brands, Inc., 954 F.3d 492, 501 (2d Cir. 2020).

"General Jurisdiction" over Dunkin' Doughnuts lacking in New York
Before 2014, "New York courts interpreted the act of registering under BCL § 1301(a) as consent to general jurisdiction in the state." See Aybar, 93 N.Y.S.3d at 169. But in 2014, the Supreme Court in Daimler AG v. Bauman, 571 U.S. 117 (2014) appears to have eviscerated that interpretation. Although the New York Court of Appeals has not "definitively weighed" in on this issue, this court "carefully predicted how the state's highest court would resolve the uncertainty or ambiguity." This court holds that "a foreign corporation does not consent to general personal jurisdiction in New York by merely registering to do business in the state and designating an in-state agent for service of process under BCL § 1301(a)."
Even if business registration did not amount to consent to jurisdiction, the plaintiffs argued, Dunkin' Doughnuts' business activities amounted to "sufficient contacts" for personal jurisdiction. But the plaintiffs made "no showing that the company's relationship with New York was in any way significant or exceptional in relation to the company's nationwide business activity." The due process clause of the 14th Amendment requires that the corporate defendant be "at home," which usually means "where it is incorporated or maintains its principal place of business"—not the case here.
Lastly, "Angus steak" is not an actionable warranty under the Magnuson-Moss Act.
Additional legal holdings cited in Chufen Chen v. Dunkin' Brands, Inc.:
- "Under New York law, as predicted by Court of Appeals, company did not consent to general personal jurisdiction in New York by registering to do business and designating an agent for service of process in the state." Chufen Chen v. Dunkin' Brands, Inc., 954 F.3d 492 (2d Cir. 2020).
- Dunkin' Brands, Inc. "could not be said to be 'at home' in New York, and thus was not subject to general personal jurisdiction in New York." Chufen Chen v. Dunkin' Brands, Inc., 954 F.3d 492 (2d Cir. 2020).
- Under New York law, a court may determine as a matter of law that an allegedly deceptive advertisement would not have misled a reasonable consumer. Chufen Chen v. Dunkin' Brands, Inc., 954 F.3d 492 (2d Cir. 2020).
- "To make out a prima facie case under Section 349, a plaintiff must demonstrate that (1) the defendant's deceptive acts were directed at consumers, (2) the acts are misleading in a material way, and (3) the plaintiff has been injured as a result." Maurizio v. Goldsmith, 230 F.3d 518, 521 (2d Cir. 2000) (citing Oswego Laborers' Local 214 Pension Fund v. Marine Midland Bank, 85 N.Y.2d 20, 25, 623 N.Y.S.2d 529, 647 N.E.2d 741 (1995)). Chufen Chen v. Dunkin' Brands, Inc., 954 F.3d 492, 500 (2d Cir. 2020).
- NYGBL § 350 "prohibits 'false advertising in the conduct of any business, trade or commerce,' and is analyzed under the same 'reasonable consumer' standard as NYGBL § 349. Id. at 521.
- "Although this Court also has the option of certifying the question to the New York Court of Appeals, certification is not warranted here because "sufficient precedents exist for us to make [the] determination." DiBella v. Hopkins, 403 F.3d 102, 111 (2d Cir. 2005). Chufen Chen v. Dunkin' Brands, Inc., 954 F.3d 492, 497 (2d Cir. 2020).
Sidenote: What is "general jurisdiction"?
"General jurisdiction" refers to a court's authority to hear and decide a wide range of cases involving a particular party, regardless of whether the specific case is related to the forum state. It grants the court broad power over a defendant, enabling it to adjudicate claims unrelated to the defendant's activities within the state.
For a court to have general jurisdiction over a defendant, the defendant must have such substantial contacts or connections with the forum state that it can be considered "at home" in that state. Traditionally, "at home" has been understood to mean a defendant's state of incorporation or principal place of business. However, general jurisdiction has evolved to include other exceptional circumstances where a defendant's contacts with the state are so extensive that it can be deemed at home there, even if it is not incorporated or headquartered in that state.
The exercise of general jurisdiction allows a court to hear and decide claims against a defendant that are unrelated to the defendant's activities within the forum state. This is in contrast to "specific jurisdiction," which relates to a court's authority to hear a case arising from or related to the defendant's contacts or activities within the state.
.png)
Sidenote: What is the Mangnuson-Moss Act?
The Magnuson-Moss Warranty Act, often called the Magnuson-Moss Act, is a United States federal law enacted in 1975. Its purpose is to regulate warranties on consumer products and provide certain rights and protections to consumers who purchase such products. It is designed to enhance consumer protection by ensuring that consumers are adequately informed about their warranty rights and providing recourse when violated. It promotes fair and honest business practices in relation to warranties on consumer products.
The Act applies to consumer products with written warranties, regardless of whether the warranties are full or limited. It does not require manufacturers to provide warranties; however, if a warranty is offered, it sets forth specific requirements for the content and disclosure of the warranty.
Key provisions of the Magnuson-Moss Act include:
- Disclosure of Warranty Terms: The Act requires manufacturers or sellers to clearly and conspicuously disclose the terms and conditions of warranties to consumers in plain language. This includes information about the duration of the warranty, what is covered and not covered, any limitations or exclusions, and the procedure for obtaining warranty service.
- Full and Limited Warranties: The Act distinguishes between full warranties and limited warranties. Full warranties provide comprehensive coverage for the repair or replacement of a product, and sellers must fulfill their obligations under these warranties. On the other hand, limited warranties may have specific conditions or limitations, but the Act requires clear disclosure of those limitations.
- Remedy Options: If a consumer product covered by a warranty is defective or fails to perform as expected, the Act provides consumers with specific options. Manufacturers or sellers are typically given an opportunity to repair or replace the defective product at no cost to the consumer. If the problem persists, the Act allows for refunds or other appropriate remedies.
- Warranty Revocation: The Act prohibits tying warranties to the use of specific products or services. Manufacturers or sellers cannot void warranties solely because a consumer used a third-party product or service that is not provided or recommended by the warrantor.
- Legal Remedies and Attorneys' Fees: The Act enables consumers to pursue legal action against manufacturers or sellers who fail to comply with its provisions. Successful plaintiffs can recover their attorneys' fees and court costs, which encourages enforcement of the Act's requirements.
Consumer plausibly alleged deception against Panera Bread for using imitation blueberries in bagels
2020: A New York man, fed up with Panera Bread's "dyed lumps" masquerading as blueberries, brought a class action. Here's the complaint.

Despite Panera's claim of "menu transparency," the plaintiff alleged the "trace amounts of real blueberries" were in "far greater proportion" than real blueberries and was, therefore, deceptive. Izquierdo v. Panera Bread Co., 450 F. Supp. 3d 453, 457 (S.D.N.Y. 2020).
Because of the blueberry trickery, the plaintiff alleges that he overpaid for the bagel. To prove his case, he introduced an image of the bagel, the ingredients list, a photograph of Panera's food display, and various web images.
Accepting the plaintiff's allegations as true, the court found that "Plaintiff plausibly alleged that a reasonable consumer is likely to be misled into believing that the Bagel's blueberry content consists solely of real blueberries, when in fact the Bagel contains primarily imitation blueberries with a lesser quantity of real blueberries."
The evidence in totality could deceive Blueberry seekers
Evidence that strengthened the plaintiff's complaint: "(1) the Bagel appears under a sign advertising Defendant's commitment to "clean food" and "menu transparency," (FAC ¶ 46; id. Ex. B); and (2) the bagel is sold alongside a Blueberry Muffin that contains only real blueberries and no imitation blueberries, (id. Ex. E)."
Panera argued that its sign reading "Blueberry" was not materially misleading because (1) the bagel does, in fact, contain blueberries, and (2) the ingredients list is readily available. (Def. 's Mem. 6–10.) "Both of these arguments fail," held the court.

Multiple district courts in this circuit, including Mantikas, have found viable claims in similar "ingredient composition" cases:
- Potentially deceptive were dog treats "marketed under a heavily branded bacon-oriented theme" contained a small amount of bacon and bacon fat but were primarily composed of "non-meat fillers. Kacocha v. Nestle Purina Petcare Co., No. 15-CV-5489 (KMK), 2016 WL 4367991, at *15 (S.D.N.Y. Aug. 12, 2016).
- "Consumers stated §§ 349 and 350 claims regarding packaging of almond milk that suggested the product "contained a significant amount of almonds," and were a "heart healthy food" when in fact they 'contained only 2% of almonds.'" Albert v. Blue Diamond Growers, 151 F. Supp. 3d 412 (S.D.N.Y. 2015.
- The packaging of personal care products that used the word "active naturals" was misleading, containing mostly synthetic and some natural ingredients. Goldemberg v. Johnson & Johnson Consumer Cos., Inc., 8 F. Supp. 3d 467, 479 (S.D.N.Y. 2014);
- "Packaging of 'vitaminwater' beverage was potentially misleading where it contained numerous statements suggesting the product was composed only of vitamins and water and that it was healthy, when in fact although the product did contain vitamins and water, it also contained a large amount of sugar, even though nutrition facts panel listed all ingredients." Ackerman v. Coca-Cola Co., No. CV-09-0395 (JG), 2010 WL 2925955, at *15–16 (E.D.N.Y. July 21, 2010).
Judge Broderick in the Southern District of New York found it "plausible that, as in Mantikas, the "Blueberry" label, in context, "falsely impl[ies] that the [blueberry] content is entirely or at least predominantly [blueberries] whereas in fact," taking plaintiff's allegations as true, the imitation blueberry component exceeds the real blueberry component."
Moreover, "the presence of accurate Nutrition Facts did not overcome misleading labeling," held J. Broderick. Mantikas "has made clear that a reasonable consumer should not be expected to consult the Nutrition Facts panel on the side of the box to correct misleading information set forth" elsewhere on the packaging." See 910 F.3d at 637.
In food-labeling cases, a well-pled "dominant-ingredient theory," tends to survive a motion to dismiss.
The plaintiff lacked standing for injunctive relief. His awareness of the blueberry issue prevents him from being legally deceived again. Injunctions address future, concrete harms—not conjectural ones.
Additional legal authorities cited in Izquierdo v. Panera Bread Co.:
- "Consistent with the black letter law, I decline to consider factual allegations and documents that are not contained in or attached to the First Amended Complaint, including Defendant's website as a whole because Defendant has not demonstrated that it is 'integral to the complaint.'" See Chambers, 282 F.3d at 152. Izquierdo v. Panera Bread Co., 450 F. Supp. 3d 453, 458 (S.D.N.Y. 2020).
- "To satisfy the requirements of standing under Article III of the Constitution, a plaintiff must establish three elements:
- "the plaintiff must have suffered an injury in fact, i.e., an invasion of a legally protected interest which is (a) concrete and particularized and (b) actual or imminent, not conjectural or hypothetical;
- there must be a causal connection between the injury and the conduct complained of; and
- The injury must be likely, and would be redressed by a favorable decision." Nat'l Org. for Marriage, Inc. v. Walsh, 714 F.3d 682, 688 (2d Cir. 2013) (quoting Lujan v. Defenders of Wildlife, 504 U.S. 555, 560–61, 112 S.Ct. 2130, 119 L.Ed.2d 351 (1992)). Izquierdo v. Panera Bread Co., 450 F. Supp. 3d 453, 459 (S.D.N.Y. 2020).
- "To demonstrate an injury in fact when seeking injunctive relief, a plaintiff cannot rely on a past injury alone,' Buonasera, 208 F. Supp. 3d at 564 (quoting *460 City of Los Angeles v. Lyons, 461 U.S. 95, 101, 103 S.Ct. 1660, 75 L.Ed.2d 675 (1983)), but must establish "a 'real or immediate threat' of injury," Nicosia v. Amazon.com, Inc., 834 F.3d 220, 239 (2d Cir. 2016) (quoting Lyons, 461 U.S. at 111–12, 103 S.Ct. 1660). Izquierdo v. Panera Bread Co., 450 F. Supp. 3d 453, 459–60 (S.D.N.Y. 2020).
- In In re Amla Litig., "Plaintiffs lacked standing to seek injunctive relief under N.Y. G.B.L. § 349 where they 'adduce[d] no evidence that they are likely to repurchase the product, and indeed allege[d] that they would not have purchased the product in the first place had they known of its alleged defects." Id. 320 F. Supp. 3d 578, 593 (S.D.N.Y. 2018).
- "The Second Circuit has found it materially misleading to suggest a product contains a greater proportion of a preferred ingredient than it actually does, even where there is a visible ingredients list that states the correct composition of the food. See Mantikas v. Kellogg Co., 910 F.3d 633, 639 (2d Cir. 2018).” Izquierdo v. Panera Bread Co., 450 F. Supp. 3d 453, 462 (S.D.N.Y. 2020).
"Cold Pressed" lemon juice involving "thermal processing" ruled potentially misleading
2018: The plaintiff sued over two "BluePrint" juice products. One is marketed as "Cold Pressed" and the other as "Organic." The cold-pressed lemon juice asserts that it is "never heated," "not cooked," and "uses pressure instead of heat to ensure food safety." The plaintiff contends that the juice underwent "thermal processing to stabilize it." Thermal processing applies heat to pasteurize or sterilize juice to help extend shelf life and kill potential pathogens. To the extent thermal processing did occur, the plaintiff stated a claim because it would involve heat.
Similarly, the defendant's organic lemon juice that is "crafted with cold pressed juice" is false for allegedly incorporating "thermal processing." The court assumed that fact true for now and let the claim proceed past a motion to dismiss.

![]()
On front labels, reasonable consumers don't expect predominant ingredients listed first.
The front label of the defendant's "Cold-Press" juice stated "beet, apple, carrot, lemon, ginger" and "raw." The "side label listed the ingredients (in order of weight) as 'organic apple, organic carrot, organic beet, filtered water, organic lemon, and organic ginger." Reasonable consumers, alleged the plaintiff, would expect beets to represent the predominant ingredient since the drink is red, the front label lists it first, and beets allegedly cost more.
But the court held that "a reasonable consumer acting reasonably under the circumstances would not understand the front label to be listing the ingredients from most to least predominant." "Consumers are accustomed to seeing a product's ingredients listed by weight under the nutrition facts—as they are here." "The ingredient lists thus clarify—in exactly the spot consumers are trained to look—that the premium ingredients are not the most predominant ingredients. No reasonable consumer could have been misled in light of this clarifying language."
The plaintiff alleged "an affirmative duty to disclose that 'the Products were processed' and that' their definition of a raw product excludes products whichare fresh.'"
Additional legal authorities cited in Davis v. Hain Celestial Group, Inc.:
- Ingredients "be listed by common or usual name in descending order of predominance by weight on either the principal display panel or the information panel." 21 C.F.R. §101.4(a)(1). Davis v. Hain Celestial Group, Inc, 297 F. Supp. 3d 327 (E.D.N.Y. 2018).
Sidenote: What is cold-pressed juice?
Cold-pressed juice uses a hydraulic press to extract the juice from fruits and vegetables. Unlike traditional juicing methods that use high-speed spinning blades, cold-pressed juicing applies pressure to crush and squeeze the produce, thereby minimizing heat and oxidation. This method helps preserve the natural enzymes, vitamins, minerals, and phytonutrients in the product, as the low heat and absence of oxygen minimize nutrient degradation.
Thermal processing would violate the cold-press technique. The use of heat is intentionally avoided to preserve the natural enzymes, vitamins, and nutrients in fruits and vegetables.
Is cold-pressed juice healthier?
Cold-pressed juice is often considered healthier than other types of juice due to its potential to retain more nutrients and enzymes. However, it's important to note that the overall healthiness of any juice depends on various factors, including the quality of the ingredients used and an individual's overall diet and lifestyle.
Here are a few factors that contribute to the perceived health benefits of cold-pressed juice:
- Nutrient retention: Cold-pressed juicing involves minimal heat and oxidation, which can help preserve the vitamins, minerals, and phytonutrients in fruits and vegetables. Since the produce is not exposed to high temperatures, higher nutrient retention is possible compared to juices made with other methods.
- Enzyme content: Cold-pressed juice is believed to contain a higher concentration of natural enzymes, as the low heat involved in the juicing process helps maintain their activity. Enzymes play a role in various bodily functions and are thought to support digestion and overall health.
- Phytonutrient availability: Phytonutrients are bioactive compounds in fruits and vegetables associated with numerous health benefits. Cold-pressed juice may retain more phytonutrients due to the gentle juicing process.
- No additives or preservatives: Cold-pressed juice is typically made from fresh fruits and vegetables without adding additives, preservatives, or artificial flavors. This can appeal to individuals seeking a more natural and unprocessed juice option.
"#1 Detergent Brand Recommended by Dermatologists for Sensitive Skin" plausibly confused the brand with the product
2017: Whether "#1 dermatologist recommended" referred to a brand or the product was not for the court to decide. The plaintiff's interpretation that it applied to the product was not "patently implausible" under Stoltz v. Fage Dairy Processing Indus., S.A., (E.D.N.Y. 2015).
In Eidelman v. Sun Products Corp., the plaintiff entered Costco with the intent to buy a chemical-free liquid laundry detergent that was "recommended by dermatologists." She purchased the "defendant Sun Product's "237-fl oz. bottle of ALL PLUS + FREE CLEAR liquid detergent." The ad claim at issue: "from the #1 Detergent Brand Recommended by Dermatologists for Sensitive Skin." It turns out that the brand—not the product—is related to the slogan. The plaintiff felt deceived by the apparent dermatological endorsement of the product. The plaintiff should not be burdened to decipher which products relate to that claim. The "tiny" prefix "from the" preceded the "#1." The plaintiff alleged that it failed to cure the misimpression that the purchased product did not fit within that brand endorsement.

It is not unreasonable for consumers to assume that the #1 dermatological claim applies to the actual product on which that advertisement sits. The court analogized cases with other plausible theories to show what should survive a dismissal:
- "Total 0%" on Greek Yogurt was plausibly interpreted as including calories, not only fat. Stoltz v. Fage Dairy Processing Indus., S.A., No. 14-CV-3826 (M.K.B.), 2015 WL 5579872, at *20 (E.D.N.Y. Sept. 22, 2015).
- "All Natural" reasonably included G.M.O. Free. In re Frito-Lay N. Am., Inc. All Nat. Litig., 12-MD-2413, 2013 WL 4647512, at *15-16 (E.D.N.Y. Aug. 29, 2013)
Here the plaintiff plausibly allegedly misleadingness, so the motion to dismiss was denied as to false advertising. But as to negligent misrepresentation, advertisements do not create a "special relationship" necessary to make a claim.
However, the plaintiff's unjust enrichment theory remained viable against Costco for its "receipt of payment" coupled with the defendant's failure to "cite a single case" dismissing such an action against the retailer for the premium paid only. "When consumers purchase a product from a third party, they confer a benefit on that third party, not on the manufacturer." See Waters v. Electrolux Home Prod., Inc., 154 F. Supp. 3d 340, 351 (N.D.W. Va. 2015).
Additional legal authorities cited in Eidelman v. Sun Products Corp.:
- Whether a representation or omission is deceptive depends upon the likelihood that it will "mislead a reasonable consumer acting reasonably under the circumstances." Cohen v. JP Morgan Chase & Co., 498 F.3d 111, 126 (2d Cir. 2007) (quoting Oswego Laborers' Local 214 Pension Fund v. Marine Midland Bank, N.A., 85 N.Y.2d 20, (1995)) ("The New York Court of Appeals has adopted an objective definition of 'misleading,' under which the alleged act must be 'likely to mislead a reasonable consumer acting reasonably under the circumstances.') Eidelman v. Sun Products Corp., 16-CV-3914 (N.S.R.), 2017 WL 4277187, at *3 (S.D.N.Y. Sept. 25, 2017).
- Generally, a misleadingness "inquiry is not appropriate on a motion to dismiss." Kacocha v. Nestle Purina Petcare Co., 15-CV-5489 (K.M.K.), 2016 WL 4367991, at *14, *16 (S.D.N.Y. Aug. 12, 2016) ("ample case law exists allowing § 349 claims over allegedly deceptively labeled consumer goods to progress beyond the motion-to-dismiss stage."). Eidelman v. Sun Products Corp., 16-CV-3914 (N.S.R.), 2017 WL 4277187, at *3 (S.D.N.Y. Sept. 25, 2017).
- "At the motion to dismiss stage, 'an action under § 349 is not subject to the pleading-with-particularity requirements of Rule 9(b) ... but need only meet the bare-bones notice-pleading requirements of Rule 8(a)....'" Kacocha v. Nestle Purina Petcare Co., 15-CV-5489 (K.M.K.), 2016 WL 4367991, at *13 (S.D.N.Y. Aug. 12, 2016) (citing Pelman ex rel. Pelman v. McDonald's Corp., 396 F.3d 508, 511 (2d Cir. 2005)).
- "Whether a reasonable consumer would be deceived by a product label is generally a question of fact not amenable to determination on a motion to dismiss." Segedie v. Hain Celestial Grp., Inc., 14-CV-5029 N.S.R., 2015 WL 2168374, at *10 (S.D.N.Y. May 7, 2015).
- "In reference to a Lanham Act claim, '[a] federal trial judge, with a background and experience unlike that of most consumers, is hardly in a position to declare that reasonable consumers would not be misled, and indicating that resolution of the issue may require "surveys, expert testimony, and other evidence of what is happening in the real world;" Verizon Directories Corp. v. Yellow Book U.S.A., Inc., 309 F. Supp. 2d 401, 407 (E.D.N.Y. 2004) Eidelman v. Sun Products Corp., at *3 (S.D.N.Y. Sept. 25, 2017).
- Applicability of unjust enrichment:
- Applied in unusual situations
- The defendant has not breached a contract nor committed a recognized tort.
- The circumstances create an equitable obligation running from the defendant to the plaintiff. Edelman at 6.
- Typical cases are those in which the defendant, though guilty of no wrongdoing, has received money to which they are not entitled." Corsello, 18 N.Y.3d at 790.
- Not available where it simply duplicates or replaces a conventional contract or tort claim. Eidelman at *6 (S.D.N.Y. Sept. 25, 2017).
Tito's "Handmade Vodka"—not made in a "hands-on, small-batch process"—is plausibly deceptive and not exempt due to license approval
2016: Tito's Handmade Vodka, which is "handcrafted" in "small batches" and "distilled six times," was challenged as false advertising. The plaintiff cites the dictionary ("made with hands or using hand tools") to establish what consumers perceive as handmade. The plaintiff alleged that Tito's vodka "is manufactured in 'massive buildings containing ten floor-to-ceiling stills and bottling 500 cases an hour using mechanized and/or automated processes, which involve little to no human supervision, assistance, or involvement."

Tito's "bottled 850,000 cases of vodka in 2012 and sold 1.2 million cases in 2013," according to the complaint. The plaintiff cited the Distilled Spirits Council of the U.S. to define 'craft spirits' as spirits produced in quantities of fewer than 40,000 cases per year."
Tito's allegedly "concealed the highly automated nature of the vodka's manufacturing and bottling process," which creates the misimpression of a "higher quality vodka." The plaintiff believed the vodka was "handmade" and "micro-distilled."
Tito's produced, and the court took judicial notice of, its Label Approvals from the U.S. Tobacco Tax and Trade Bureau (T.T.B.) and the New York State Liquor Authority. These approvals qualify Tito's for Safe Harbor relief under New York General Business law § 349(d), which affords a "complete defense" for any act or practice that complies with federal law, argued the plaintiff. In a classic preemption battle, Tito's argued that the U.S. Tobacco Tax and Trade Bureau had already approved the label as non-misleading. In response, the plaintiff argued that "the fact that Tito's label has not been rejected does not mean that the T.T.B. has expressly authorized the misrepresentations on the label."
The safe-harbor carve-outs have produced mixed results in analogous court holdings in other jurisdictions. For example, in Hofmann v. Fifth Generation, Inc. (S.D.Cal. 2015), the court declined to apply the California safe harbor since the T.T.B. was not shown to have "specifically investigated and approved the 'handmade' statement." Similarly, in Aliano v. WhistlePig, L.L.C., a "bottle label was misleading because it stated that the whiskey was "hand-bottled" in Vermont whereas it was in fact mass-produced in Canada." Aliano found nothing suggests that the "T.T.B. label approval process is akin to those in the highly regulated tobacco, food and drug industries."
Here, the Tito's court found that the U.S. Trade Bureau's approval was not specific enough for safe-harbor protection, unlike its "Gluten-free" approval based on a "specific T.T.B. ruling, pending rulemaking on gluten-free references by the F.D.A." Nor is there a federal regulation for the T.T.B. to interpret § 349(d) to define the terms "handmade" and "crafted in an old-fashioned pot still."

"Moreover," held the court, "the T.T.B. application form states that the issuance of a certificate does not relieve Defendant from liability for violations of the Federal Alcohol Administrative Act ("F.A.A. Act"), which itself prohibits false and misleading labeling, 27 U.S.C.A. § 205(e), suggesting that T.T.B. approval is not intended to carry pre-emptive weight."
Drawing all reasonable inferences in favor of the plaintiff, the court found that reasonable consumers could interpret "handmade" as a "hands-on, small batch process" without "complex automated machinery."
Other legal authorities cited in Singleton v. Fifth Generation, Inc.:
- "In considering a motion to dismiss for failure to state a claim under Fed.R.Civ. P. 12(b)(6), a district court must limit itself to facts stated in the complaint or in documents attached to the complaint as exhibits or incorporated in the complaint by reference." Kramer v. Time Warner Inc., 937 F.2d 767, 773 (2d Cir.1991). Singleton v. Fifth Generation, Inc., 515CV474BKSTWD, 2016 WL 406295, at *2 (N.D.N.Y. Jan. 12, 2016).
- "Under Rule 201(b)(2), the Court may take judicial notice of a fact that is not subject to reasonable dispute because it 'can be accurately and readily determined from sources whose accuracy cannot reasonably be questioned.'" Fed.R.Evid. 201(b)(2).
- "The Court may take judicial notice of such facts on its own, and "must take judicial notice if a party requests it and the court is supplied with the necessary information." Fed.R.Evid. 201(c).
- "Matters of public record are suitable for judicial notice, see e.g., Giraldo v. Kessler, 694 F.3d 161, 164 (2d Cir.2012), including administrative decisions by federal regulatory agencies. See Colon v. Holdridge, No. 9:13–CV–1546 DNH/ATB, 2015 WL 1730240, at *4, 2015 U.S. Dist. LEXIS 48528, at *10 (N.D.N.Y. Apr. 14, 2015) ("the court may consider matters of which judicial notice may be taken, such as public filings and administrative decisions").
- It is well-established that paying a premium for a product can constitute an actual injury under N.Y. G.B.L. § 349. See, e.g., Koenig v. Boulder Brands, Inc., 995 F.Supp.2d 274, 288 (S.D.N.Y.2014). Moreover, at the pleading stage, it is not necessary to specifically identify the amount of the premium based on prices of competing products. See Goldemberg, 8 F.Supp.3d at 481–482 Singleton v. Fifth Generation, Inc., 515CV474BKSTWD, 2016 WL 406295, at *11 (N.D.N.Y. Jan. 12, 2016).
- “The primary reason for requiring notice is to give the seller [of breach of express warranty] the opportunity to make adjustments or replacements, opportunities to minimize the buyer's loss and reduce the seller's own liability.” Besicorp Group, Inc. v. Thermo Electron Corp., 981 F.Supp. 86, 101–102 (N.D.N.Y.1997). “The second reason for requiring notice is to give the seller a chance to prepare for negotiation and litigation.” Singleton v. Fifth Generation, Inc., 515CV474BKSTWD, 2016 WL 406295, at *12 (N.D.N.Y. Jan. 12, 2016).
- “The failure to oppose a motion to dismiss a claim is deemed abandonment of the claim ... and, in the Northern District of New York, is deemed consent to granting that portion of the motion.” Barmore v. Aidala, 419 F.Supp.2d 193, 201–02 (N.D.N.Y.2005).
- “The elements of common-law fraud and intentional misrepresentation under New York law are the same.” B & M Linen, Corp. v. Kannegiesser, USA, Corp., 679 F.Supp.2d 474, 480 (S.D.N.Y.2010) (citing Indep. Order of Foresters v. Donald, Lufkin & Jenrette, Inc., 157 F.3d 933, 940 (2d Cir.1998)). “In either case, a plaintiff must show that: '(1) the defendant made a material false statement or omission; (2) the defendant intended to defraud the plaintiff; (3) the plaintiff reasonably relied upon the representation or omission; and (4) the plaintiff suffered damage as a result of such reliance.' Singleton v. Fifth Generation, Inc., 515CV474BKSTWD, 2016 WL 406295, at *14 (N.D.N.Y. Jan. 12, 2016).
Nature's Bounty "Black Cohosh" supplement for menopausal symptoms ruled possibly misleading
2016: Black Cohosh plant marketed by Nature's Bounty as "Natural Whole Herb" and "Natural Menopausal Relief" was plausibly misleading because they contained "unnatural" ingredients like lead and magnesium stearate, alleged the plaintiff.

The court agreed, denying the defendant's motion to dismiss under New York General Business Law § 349.
The parties disputed Nature Bounty's obligation to prove the product's impact on menopausal symptoms. The plaintiff contended that her complaint invoked sufficient scientific support to debunk the efficacy of Black Cohosh. It is "no better than a placebo," argues the plaintiff. Nature's Bounty contended that she needed to "disprove" its menopausal claims specifically.
But such "issues of fact, credibility, and the weight of the evidence are not properly considered on a motion to dismiss." Kardovich, 97 F. Supp. 3d at 140, which is consistent with other courts in adjudging disputed health claims:
- "Whether or not the studies support plaintiff's proposition that it is 'biologically impossible' to rebuild cartilage is an issue of fact the court cannot resolve on a motion to dismiss." Quinn, 958 F. Supp. 2d 533, 544 (S.D.N.Y. 2013);
- Facially plausible scientific studies tending to rebut representations as "provably false is a question not properly decided on a motion to dismiss." Vasic v. Patent Health, LLC., No. 13-CV-849, 2014 WL 940323, at *4 (S.D. Cal. Mar. 10, 2014).
Neither the defendant's alleged compliance with FDA regulations nor an accurate nutrition label foreclosed a consumer-deception claim. A consumer could reasonably conclude that the product contains only herbs and spices and no lead, as alleged by the plaintiff. The case is Sitt v. Nature's Bounty, Inc., 15-CV-4199 (MKB), 2016 WL 5372794, at *1 (E.D.N.Y. Sept. 26, 2016).
.png)
Sidenote: What is Black Cohosh?
Black Cohosh (Actaea racemosa) is a plant native to North America. It is a member of the Buttercup family and is recognized by its tall white flowers.
The root of the black cohosh plant has been used for medicinal purposes by Native American cultures for centuries and later by European settlers. Today, it is commonly used as a dietary supplement for menopausal symptoms, including hot flashes, mood changes, excessive sweating, and improved sleep.
Scientific research has been somewhat mixed regarding the effectiveness of black cohosh for these uses. As with all dietary supplements, it can interact with other medications and cause side effects. Some potential side effects may include gastrointestinal upset, rashes, a feeling of heaviness in the legs, and, in rare cases, liver damage.
As of 2023, the FDA has not approved black cohosh for any medical use due to insufficient evidence of its effectiveness, safety concerns, and the potential for product adulteration in the marketplace.
Additional legal authority cited in Sitt v. Nature's Bounty, Inc.:
- "The Second Circuit has recognized that "in determining whether a reasonable consumer would have been misled by a particular advertisement, context is crucial," and, "under certain circumstances, the presence of a disclaimer or similar clarifying language may defeat a claim of deception." Fink, 714 F.3d at 742. However, "the presence of a nutritional panel, though relevant, does not as a matter of law extinguish the possibility that reasonable consumers could be misled by [the defendant] 's labeling and marketing." Ackerman, 2010 WL 2925955, at *16. Sitt v. Nature's Bounty, Inc., 15-CV-4199 (MKB), 2016 WL 5372794, at *12 (E.D.N.Y. Sept. 26, 2016).
- "The mere inclusion of an accurate disclaimer does not necessarily cure other potentially misleading statements or representations in a label or advertisement." See Goldemberg, 8 F. Supp. 3d at 478–481 (Synthetic ingredients listed did label did not cure the misimpression of "Active Naturals" claims). Hughes, 930 F. Supp. 2d at 463–65 (FDA disclaimer did not necessarily fix the misimpression of a cold or flu prevention product. See In re Frito-Lay N. Am., Inc. All Nat. Litig., No. 12-MD-2413, 2013 WL 4647512, at *23–24 (E.D.N.Y. Aug. 29, 2013) (An explanatory ring of text stating "no MSG — no preservatives — no artificial flavors" that surrounded the words "all natural," did not obliterate the idea that reasonable consumers would expect GMO-Free); see also Williams v. Gerber Prods. Co., 552 F.3d 934, 939–40 (9th Cir. 2008) ("Reasonable consumers should [not] be expected to look beyond misleading representations on the front of the box to discover the truth from the ingredient list in small print on the side of the box."). Sitt v. Nature's Bounty, Inc., 15-CV-4199 (MKB), 2016 WL 5372794, at *13 (E.D.N.Y. Sept. 26, 2016).
- “The MMWA merely incorporates and federalizes state-law breach of warranty claims.” Sitt v. Nature's Bounty, Inc., 2016 WL 5372794, *17 (E.D. N.Y. 2016). § 21:7. Magnuson-Moss Warranty Act (MMWA), 7 Newberg and Rubenstein on Class Actions § 21:7 (6th ed.).
- "An express warranty is not a generalized statement such that a reasonable consumer would not interpret the statement as a factual claim upon which he or she could rely.” Sitt v. Nature's Bounty, Inc., Case No. 15-cv-4199 (MKB), 2016 WL 5372794, at *15 (E.D.N.Y. Sept. 26, 2016). Newton v. Kraft Heinz Foods Co., 16CV04578RJDRLM, 2018 WL 11235517, at *7 (E.D.N.Y. Dec. 18, 2018)
Plaintiffs plausibly allege that using GMOs in "all natural" Sun Chips is deceptive
2013: Sun Chips and Tostitos labeled as "natural" and "all-natural" have allegedly crossed the line since they incorporate genetically modified organisms (GMOs). With numerous "follow-on" suits in other federal districts disputing "all natural," this court (EDNY) consolidated this action. The court decided thirteen causes of action involving consumer laws of three states (New York, California, and Florida). The case is In re Frito-Lay NA, Inc. All Nat. Litig., (E.D.N.Y. Aug. 29, 2013).
"Here, the Tostitos and Sun Chips products label include an explanatory ring surrounding the "Made with ALL NATURAL ingredients" center. That ring states, "No MSG—No Preservatives—No Artificial Flavors." Indeed, these additional representations give context to the label's center pronouncement. "But to hold as a matter of law that they show no reasonable consumer would be deceived into believing the product is GMO-free is not a conclusion the Court can reach at this stage."


Frito-lay introduced various studies showing that three federal agencies, states, and consumer groups agree that "natural does not mean GMO-free." But the court also noted, "in the FDA's view, a 'general lack of consumer understanding and scientific agreement about the meaning of the term.'" "...Whether a reasonable consumer would likely be deceived by the designation 'All Natural' is a factual dispute." Vicuna v. Alexia Foods, Inc., N.D.Cal. Apr. 27, 2012. "Therefore, it is generally not resolved on a motion to dismiss." Jones v. ConAgra Foods, Inc., 912 F.Supp.2d 889, 899 (N.D.Cal.2012);
FDA non-binding guidance about "all natural" did not preempt the plaintiff's consumer claims. For an exhaustive look at preemption, see this blog: Does Federal Law "preempt" (Block) State Lawsuits for Mislabeling?
PepsiCo, the parent company to Frito-Lay, was an improper defendant since it played no part in marketing the chips as "all natural."
Additional legal authority cited in In re Frito-Lay NA, Inc. All Nat. Litig.:
- "A district court may consider matters of which judicial notice may be taken without converting a motion to dismiss into one for summary judgment." Staehr v. Hartford Fin. Servs. Grp., Inc., 547 F.3d 406, 425 (2d Cir.2008).
- "Courts may judicially notice facts that are "not subject to reasonable dispute because [they] ... can be accurately and readily determined from sources whose accuracy cannot reasonably be questioned." Fed.R.Evid. 201(b)(2).
- "Whether to take judicial notice is an inquiry separate and distinct from what inferences or conclusions to draw from the noticed material." See Staehr, 547 F.3d at 426 (affirming district court's decision to take judicial notice of media reports, state court complaints, and regulatory filings, but reversing its conclusion that noticed information was sufficient to rule, as a matter of law, that investors were on inquiry notice of allegedly fraudulent conduct).
- The contents of the Federal Register shall be judicially noticed." 44 USC § 1510.
- The court took notice of agency guidance documents, consumer advisory, backgrounder, and letters because they were available on the FDA website. Porazzo v. Bumble Bee Foods, LLC, 822 F.Supp.2d 406, 411–12 (S.D.N.Y.2011).
- In failure to warn and strict liability cases, parent companies are not liable if they do not manufacture the product at issue. See Magnus v. Fortune Brands, Inc., 41 F.Supp.2d 217, 224 (E.D.N.Y.1999). "A parent company 'necessarily exercise[s] a considerable degree of control over the subsidiary corporation.'" "The discharge of that supervision alone" is not enough to give rise to direct liability. Volkswagenwerk Aktiengesellschaft v. Beech Aircraft Corp., 751 F.2d 117, 120 (2d Cir.1984).
- "The 'All Natural' label on the various chip and dip products does not constitute a written warranty as defined by the MMWA. "An 'All Natural' label does not warrant a product free from defect. Wilson v. Frito–Lay N. Am., Inc., No. 12–CV–1586, 2013 WL 1320468, at *15 (N.D.Cal. Apr. 1, 2013). In re Frito-Lay NA, Inc. All Nat. Litig., 12-MD-2413 RRM RLM, 2013 WL 4647512, at *1 (E.D.N.Y. Aug. 29, 2013).
Postponing a Rolling Stones concert was not deceptive, fraudulent, or a breach a contract
2007: Aggrieved ticket holders of a Rolling Stones concert sued over a sudden cancellation of a concert in Atlantic City. For an untimely cancellation, the plaintiff sued TicketMaster, Mick Jagger, The Rolling Stones, Live Nation, and the Borgata Hotel. The plaintiff theorized that the defendants fraudulently withheld cancellation notice until 4 pm on the day of the concert and did so for financial or self-interested reasons. The case is Druyan v. Jagger, 508 F. Supp. 2d 228, 244 (S.D.N.Y. 2007).

![]()
The tickets themselves and the website's "terms of use" both disclaimed incidental damages, like traveling costs
However, the tickets contained a disclaimer "DATE & TIME ARE SUBJECT TO CHANGE." The "Terms of Use" on the website were "absolutely silent" on the issue of when to provide notice of cancellation. The "Terms of Use" were held to be 'sufficiently conspicuous" to be enforceable in the contract. The ticket itself, too, is an enforceable contract. Both contracts disclaimed any guarantee of performance, and both were silent regarding the timing of cancellations. Ticketmaster expressly disclaimed liability for "travel expenses, any indirect, consequential, exemplary, incidental, special, or punitive damages under any circumstances." The court found that internet advertisements and daily emailed updates by Ticketmaster did not amount to a guarantee of adequate notice of cancellation.
Perhaps harshly, the court found that the plaintiff had "no legitimate expectation that she would be apprised of any change in scheduling at any point in time leading up to the Concert."
Here is the court's rendition of Ticketmaster's disclaimer:
"To purchase her tickets from agency, user was required to click on a "Look for Tickets" button, immediately above which appeared a statement that by clicking on the button "or otherwise using this web site, you agree to the Terms of Use," and clicking on "Terms of Use" link presented the full terms of use, including agency's "Purchase Policy" and a link directly to that policy, and agency's "Cancelled/Postponed Events" policy and a link directly to that policy."
To assert fraud or deception, plaintiffs need more than a breach of contract
This was a contract case. A fraud theory cannot be predicated on the duties arising from the existing contract. A plaintiff would need to allege activity "collateral or extraneous to the contract when seeking "special damages that are caused by the misrepresentation and unrecoverable as contract damages." The court was not convinced that the plaintiff had justifiably relied on a "concealment" of the postponement. Nor did the plaintiff establish a "fraudulent scheme."
The plaintiff's travel expenses were not "special damages" arising from a prima facie tort
The defendants offered to refund the money or grant access to another show. The plaintiff sought more in the form of "special damages," such as traveling and incidentals. The court found the plaintiff's costs to be "incidental"—not "special" that would enable recovery under a theory besides breach of contract.
Additional legal authorities cited in Druyan v. Jagger:
- "Under New York law, the use of a web site for such purposes as purchasing a ticket to a concert manifests the web site user's assent to the web site's terms of use, and such terms constitute a binding contract between the user and the web site operator as long as the terms are sufficiently conspicuous." Druyan v. Jagger, 508 F. Supp. 2d 228 (S.D.N.Y. 2007).
- "Under New York law, a plaintiff cannot plead a fraud claim based on the same facts that underlie her breach of contract claim, unless she: (1) demonstrates a legal duty separate from the duty to perform under the contract; (2) demonstrates a fraudulent misrepresentation collateral or extraneous to the contract; or (3) seeks special damages that are caused by the misrepresentation and unrecoverable as contract damages." Druyan v. Jagger, 508 F. Supp. 2d 228 (S.D.N.Y. 2007).
- "Under New York law, the implied covenant of good faith and fair dealing does not create any new contractual rights or provide an independent basis for recovery."
- "To state a cause of action under New York law for fraud based on concealment, a plaintiff must allege
- a misrepresentation or material omission of fact which was false and was known to be false by the defendant,
- made to induce the plaintiff to rely upon it,
- plaintiff's justifiable reliance on the misrepresentation or material omission, and
- injury. Druyan v. Jagger, 508 F. Supp. 2d 228 (S.D.N.Y. 2007).
- Under New York law, a cause of action sounding in prima facie tort consists of four elements:
- intentional infliction of harm:
- causing special damages;
- without excuse or justification;
- by an act or series of acts that would otherwise be lawful. Druyan v. Jagger, 508 F. Supp. 2d 228 (S.D.N.Y. 2007).
- The prima facie tort cause of action exists under New York law to provide redress for acts that, while otherwise lawful and not encompassed by a traditional tort, are done solely for an improper or evil motive. Druyan v. Jagger, 508 F. Supp. 2d 228 (S.D.N.Y. 2007).
.png)
2023
Ambiguity in the amount of Omega-3s in Whole Foods' Fish Oil was not necessarily misleading
2022
Environmental impact and animal welfare claims were deemed not materially misleading
Specific, actionable misrepresentations not asserted against Target's "Toddler Next Stage" drink
FDA warning letter over unregulated Phenibut did not prove a false-advertising case
2021
Smokehouse® almonds may misleadingly imply that they are smoked over a fire
"SPARKLING MARGARITA" and "LIME-A-RITA" may deceptively imply the inclusion of alcohol
Whole Foods' "Oats & Flax" is not misleading as to whole-grain content or sugar content
An incomplete legal treatise is “consumer-oriented” but not “materially misleading”
Weight-loss product not “materially misleading” for not disclosing alleged cancer risk
2020
Godiva Chocolate, marketed as "Belgium 1926," reasonably implied that it was made in Belgium
Some animal-welfare claims on egg cartons were fair game in a consumer-fraud case
Wegmans did not deceptively imply the source of vanilla flavoring in its ice cream
2019
2018
"Natural" cosmetics are possibly deceptive when they contain synthetic ingredients
Lawsuit over false "run resistant" L'eggs stockings proceeds; Similar Amazon reviews were considered
Dr. Scholl's Foot Mapping Kiosks were not misleading, held the court
2017
Plaintiffs failed to prove that Dannon’s “natural” yogurt was not “natural”
Evidence of fraudulent intent against Gerber for false allergy claims
2016
Unapproved allergy-reduction claims on infant formula were false and deceptive, alleged class action
2015
Consumers plausibly alleged that Staple's "Carry-in Protection Plan" is deceptive in scope
"Total 0%" on Greek yogurt lacked clarification and was arguably deceptive under New York law
2013
Plaintiffs must produce accurate versions of the advertisements at issue
2012
Justifiable reliance on a false-advertising claim is not required under NY law
2002
Hewlett-Packard's half-ink cartridges were not materially misleading
2000
A bank fee for processing a mortgage refinance was not a deceptive prepayment charge
1999
False advertising claims against an In vitro fertilization company may proceed
"Vanishing premiums" in insurance contracts were possibly deceptive but not fraudulent
1996
1995
Bank's dealings with non-profits' pension investments declared "consumer-oriented"
Related links:
- To get a free evaluation of your false-advertising case, complete this form.
- To understand whether federal law preempts state law on labeling of food, dietary supplements, or medications, click Does Federal Law "Preempt" (Block) State Lawsuits for Humane-Washing of Meat Labels?
- Learn more about Vitamins & Dietary Supplements
- For an expansive look into humane-washing, click Is Humane-Washing of Meat and Poultry False Advertising?
- Learn about the 4 purposes of advertising, along with numerous common myths in false adverting.
- For a comprehensive review of New York law, click The Ultimate Guide to False-Advertising Law in New York: 22 FAQs.
- Click here to learn more about humane-washing, green-washing, and nutri-washing.
- Were you Misled?: Complete that form for a free legal analysis to determine if you were deceived by humane-washing or green-washing.
- A library of links relating to nutrition, animal welfare, and advertising law.
To speak with Jesse Langel personally about these types of claims, contact him or complete this tailored questionnaire.