In general, mislabeling refers to errors or omissions on product labels, while misbranding refers to false or misleading claims or representations about the product.
Quick Examples of Mislabeling:
- A food product is labeled with an incorrect "sell by" or "use by" date.
- An ingredient is not listed on a product label, even though it is required by law.
- A food product is labeled as “all natural” but contains a synthetic preservative.
- A product label contains a typo or misspelling.
- A food product is labeled as "gluten-free" even though it contains gluten.
- A juice drink is marketed as “100% pure” while it contains added sugars and flavors.
- A product label includes incorrect information about the amount or concentration of an ingredient.
- A product is labeled with an inaccurate weight or volume measurement.
- The font size on a product label is too small to be easily read.
- A product label does not include required warnings, such as a warning that a product contains an allergen.
- A label that does not include required information, such as the net weight or the name and address of the manufacturer or distributor.
Quick Examples of Misbranding:
- A dietary supplement is labeled with false or misleading claims about its benefits, such as claiming that it can cure a disease.
- An “organic” supplement that contains non-organic ingredients is misbranded.
- A food product is labeled as "organic" despite not meeting the legal requirements for organic certification.
- A dietary supplement that contains a drug not approved by the FDA is misbranded.
- A cosmetic product is labeled as "hypoallergenic" even though it contains ingredients that are known to cause allergic reactions in some people.
- A product is labeled as "made in the USA" even though some of its components or ingredients are sourced from other countries.
- A weight loss product is labeled with false or misleading claims about its effectiveness, such as claiming that users can lose 10 pounds in a week without changing their diet or exercise habits.
- A product is marketed as a medical device without the appropriate regulatory clearance or approval from the relevant authorities.
- A company uses false or misleading before-and-after photos that are not representative of typical results.
- A food product is marketed as "low-fat" even though it contains high amounts of sodium or other unhealthy ingredients.
- Otherwise, misrepresenting nutrients that do not comply with 21 U.S.C. § 343(r)(2).
What are The Four types of Labels?
- Brand labels: Contains only the brand name, such as Dove or Olay.
- Informative labels: Provides product information like the manufacturer’s name, manufacture, and expiration dates, intermediary suppliers, and additional instructions on the product’s usage. Disposal and recycling processes may be on the label. Potentially poisonous chemicals need a warning. Informative labels are generally more detailed than descriptive labels.
- Descriptive labels: Communicate information about the product’s attributes. It may explain how to use the product, its features, handling (onloading, uncrating, proximity to other substances), security, and storage.
- Grade labels: Grades by level or distinction of the product’s features and aspects. Gradation is measured in figures, letters, or words. For example, A, B, C, D or 1, 2, 3, 4, or Good, Better, Best. Grade levels assist with quality distinction.
What is Product Labeling, and why is it Important?
Labeling itself means any words, particulars, trademarks, brand name, pictorial matter, or symbol relating to food and placed on any packaging, document, notice, label, ring, or collar accompanying or referring to such food.[1] A label can be affixed to a textile (clothing, fabric, accessory) product.
Broadly speaking, labeling means information on the immediate or outer packaging.[2] Labeling assists in accurate and transparent comparisons and buying decisions. Uniform and accurate labeling contributes to supply-chain efficiency and counterfeit mitigation.
Labeling warns the public of allergens and other ingredients that could be harmful. Labels communicate the product’s composition so consumers can be aware of harmful allergens. In medicinal products, labels provide information for their safe use to avoid complications. Some labels need to warn about susceptible members of the public, such as children.
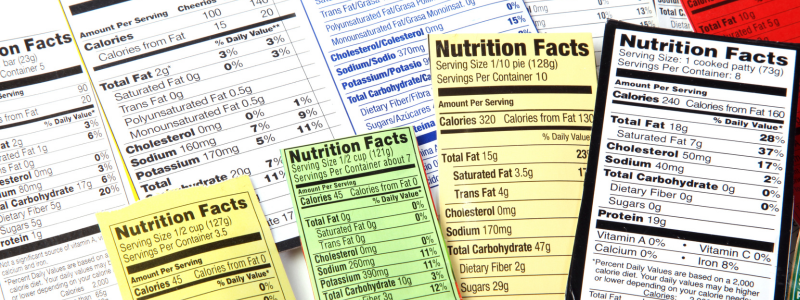
With food items, the Nutrition Facts table on food and beverages helps consumers seek out healthier options. Nutrition labels contain information such as vitamins, minerals, calories, carbohydrates, calcium, iron, protein, and fat quantities.
Compliance with labeling laws is a must. Food, supplements, medical devices, cigarettes, over-the-counter medicine, cosmetics, and more are governed by different regulatory standards.
What is Mislabeling?
Mislabeling generally refers to a product that contains an incorrect label.
Mislabeling may misrepresent ingredients, benefits, risks, and compliance with laws, including formatting such as type, placement, and font.
What are Ten Types of Mislabeling
- Food mislabeling: When food packaging does not accurately reflect the product's contents, such as a vegetarian dish that contains meat or a product labeled as "organic" but does not meet organic standards.
- Product mislabeling: When a product is labeled as something it is not, such as a synthetic leather product being labeled as "genuine leather."
- Cosmetic mislabeling: When cosmetic products are labeled with false or misleading claims, such as a "natural" product containing synthetic ingredients.
- Pharmaceutical mislabeling: When the label on a medication does not accurately reflect the ingredients or dosage, potentially causing harm or adverse effects.
- Environmental mislabeling: When products are labeled as environmentally friendly but are not sustainable or negatively impact the environment.
- Country-of-origin mislabeling: When a product is labeled as being from a certain country but was actually manufactured in a different country.
- Nutritional mislabeling: When the nutritional information on a food product is inaccurate or misleading, such as a product claiming to be "low fat" but actually containing high levels of sugar.
- Weight mislabeling: When the weight listed on a product is incorrect, such as a bag of coffee that is supposed to be 12 ounces but actually contains only 10 ounces.
- Expiration date mislabeling: When the expiration date on a product is incorrect or has been intentionally altered.
- Animal welfare mislabeling: When food products are labeled as being from animals raised in humane conditions but are actually from animals that were raised in inhumane conditions.
Ten Companies Accused of Mislabeling
- Volkswagen: In 2015, Volkswagen was found to have intentionally mislabeled their diesel vehicles as having lower emissions than they actually did, leading to a scandal known as "Dieselgate."
- Subway: In 2014, Subway faced criticism after it was discovered that their "footlong" sandwiches were actually only 11 inches long.
- Enron: Enron, a former energy company, was found to have arguably mislabeled its financial statements and engaged in accounting fraud in the early 2000s, leading to one of the largest corporate scandals in history.
- Herbalife: Herbalife, a nutritional supplement company, was fined $200 million in 2016 for mislabeling their products as healthy and safe when they were actually causing liver damage.
- Fyre Festival: In 2017, the Fyre Festival was heavily criticized for mislabeling their luxury music festival as a high-end experience, when in reality it was poorly planned and disastrous.
- PepsiCo: In 2011, PepsiCo agreed to pay $5 million for mislabeling their Naked Juice products as "all-natural," when they actually contained synthetic ingredients. The lawsuit alleged that the products contained ingredients such as a fiber additive made by Archer Daniels Midland and genetically modified soy, which are not considered natural.
- Amazon: In 2019, Amazon faced criticism for mislabeling their own brand of earbuds as "AirPods" in their search results, leading to confusion for customers. Amazon's search algorithm was reportedly displaying search results for "AirPods" that included listings for the Amazon Echo Buds, the company's own brand of earbuds.
- Samsung: In 2016, Samsung was found to have mislabeled the battery capacity of their Galaxy S7 phones, leading to complaints from customers who found that the battery life did not meet their expectations.
- Chipotle: In 2015, Chipotle agreed to pay $6.5 million for allegedly mislabeling their food as "GMO-free," when some of their ingredients actually contained genetically modified organisms. It was discovered that some of the ingredients, such as the cooking oil and the dairy products used in their food, did contain genetically modified organisms, even though they had been labeled GMO-free.
- Tesla: In 2020, Tesla was accused by the California Department of Motor Vehicles of having mislabeled their Autopilot feature, which is not actually a fully self-driving system, leading to confusion for some customers who believed the feature was more advanced than it actually was.

7 Companies Accused of Mislabeling Dietary Supplements
- GNC: In 2015, GNC was fined $2.25 million for mislabeling certain supplements, including a product that was marketed as containing an herbal extract that had been spiked with synthetic drugs.
- USPLabs: In 2015, USPLabs was indicted for selling dietary supplements containing a synthetic stimulant that had been mislabeled as an herbal extract.
- Hi-Tech Pharmaceuticals: In 2018, Hi-Tech Pharmaceuticals was fined $40 million (later vacated by the Eleventh Circuit) for mislabeling and selling illegal dietary supplements containing a stimulant that had been banned by the FDA. The stimulant in question is called DMAA (1,3-dimethylamylamine), which was commonly used in dietary supplements as a weight loss and athletic performance enhancer. However, in 2012, the FDA issued warning letters to companies selling DMAA supplements, stating that the substance was unsafe and could lead to serious health problems, including heart attacks and strokes.
- Jack3d: Jack3d was a popular pre-workout supplement that was found to contain a dangerous stimulant that had been mislabeled as an herbal extract. The product was eventually banned by the FDA. Jack3d also contained the dangerous stimulant called DMAA (1,3-dimethylamylamine), which was mislabeled as an herbal extract on the product's label. The product was eventually banned by the FDA in 2013.
- Bodybuilding.com: In 2012, Bodybuilding.com was fined $7 million for selling mislabeled dietary supplements that contained ingredients not listed on the label. The investigation found that Bodybuilding.com had sold 43 products that contained ingredients not listed on the label, including anabolic steroids, synthetic stimulants, and other prescription drugs. These products were sold under various brand names, and their mislabeling led to consumers being exposed to potentially dangerous substances without their knowledge.
- Nutrex: In 2012, Nutrex was sued in a class action for mislabeling a fat-burning supplement that contained the dangerous stimulant DMAA.
- MusclePharm: In 2015, MusclePharm was sued for mislabeling a pre-workout supplement over an alleged use of ingredients not approved by the FDA.
What is Misbranding?
Misbranding refers to any written, printed, or graphic matter on products, labels, or containers. Misbranding encompasses labels and outer packaging. Misbranded labels may not contain legally required information such as the name, manufacturer, or distributor. A misbranded label may misrepresent quality, origin, or some other product attribute.
Misbranding may misrepresent a product’s ingredients, benefits, or safety.
Misbranding may refer to unsubstantiated claims, misinformation, or misleading information on the label or other information materials, including those contained in brand names or trademarks. Misbranding can reach promotional materials, websites, digital infomercials, and campaigns.
Misbranding may cause confusion in the market or in comparison to other products. It refers to any branding or marketing that is misleading or deceptive in some way. It's often calculated to mislead reasonable consumers acting reasonably.
Can a Deceptive Label Amount to a Misbranded Product?
Yes, a deceptive label can cause a product to be considered misbranded. In the context of consumer products, particularly food and drugs, a product may be deemed misbranded if its labeling is false or misleading in any particular way.
According to the U.S. Food and Drug Administration (FDA), a food product can be considered misbranded if its label is false or misleading, fails to include the required information, or does not comply with specific regulations regarding the product's name, ingredients, or other mandatory labeling requirements.
Similarly, under the Federal Food, Drug, and Cosmetic Act (FDCA), a drug can be considered misbranded if its labeling is false or misleading, does not contain adequate directions for use, lacks adequate warnings, or does not comply with other specific regulations.
In both cases, labeling requirements and regulations aim to ensure that consumers have accurate and reliable information about the products they purchase, allowing them to make informed choices. A deceptive label that misrepresents a product's contents, benefits, or risks can lead to a product being considered misbranded and subject to regulatory action, such as recalls, fines, or other penalties.

Legal Consequences for Misbranding and Mislabeling
The legal consequences for misbranding can vary depending on the severity of the violation, the product involved, and the relevant regulations and laws in the jurisdiction where the misbranding occurred. Here are some potential consequences of misbranding:
- Civil penalties: Companies that engage in misbranding may be subject to civil penalties, which can include fines, product recalls, and mandatory corrective action to bring the product into compliance.
- Criminal charges: In some cases, misbranding can result in criminal charges against the company or individuals involved. This is more likely to occur in cases of intentional misbranding, such as when a company knowingly and willfully makes false or misleading claims about a product.
- Lawsuits: Misbranding can also result in lawsuits from consumers who were harmed or deceived by the product. These lawsuits can result in financial damages to the company and damage the company's reputation and brand.
- Regulatory action: In addition to civil and criminal penalties, regulatory agencies such as the FDA or FTC may take action against companies that engage in misbranding. This can include fines, injunctions, and other actions to bring the company into compliance with regulations.
- Loss of business: Misbranding can result in loss of business as consumers lose trust in the company and choose to buy from competitors instead. This can have long-term financial consequences for the company and damage its reputation and brand.
What are 10 Types of Misbranding?
- Unapproved claims: Any claims, whether on or off-label, especially medical or scientific claims, which have not obtained requisite approval from any regulatory agency. Supplements and other health products are often rife with unapproved health claims.
- Unsupported health claims: Related to unapproved claims, A product or claim is misbranded if it claims to cure or treat a disease but lacks competent and reliable scientific evidence to support it.
- Misleading advertising: Any medium of marketing communication (web, print, social media, podcast, etc.) that misrepresents a product, product type, company, or claim that deceptively represents benefits, safety, or efficacy.
- Confusing labeling: A product that has labeling that is likely to be confused with another product.
- Lack of warning label: A product that poses a safety risk but does not have an appropriate warning.
- Counterfeit product: A product that is falsely labeled as a well-known brand, leading to consumer deception.
- Imitation of another product: A product that imitates the name, packaging, or labeling of another product, leading to consumer confusion in the marketplace.
- Incomplete labeling: A label that is missing required information, such as the name and address of the manufacturer.
- Inaccurate labeling: A label that lists incorrect ingredients or dosages.
- Misleading packaging: The product packaging (outside of the label) that makes false or misleading claims about the product's benefits, safety, or efficacy.
Where does Misbranding Occur?
- Product labeling: Misbranding can occur if the label on a product is false or misleading in any way. This can include incorrect or incomplete ingredient lists, misleading claims about the product's effectiveness or benefits, or inaccurate information about the product's origin or manufacturing process.
- Advertising and marketing: Misbranding can also occur in the advertising and marketing of a product. This can include making false or misleading claims about the product's effectiveness, benefits, or safety.
- Product packaging: Misbranding can occur if the packaging on a product is false or misleading in any way. This can include incorrect or incomplete information about the product or using packaging that is intended to deceive or mislead consumers.
- Manufacturing and production: Misbranding can occur if a product is not manufactured or produced in accordance with the regulations and standards set by the relevant governing bodies. This can include using unapproved ingredients or manufacturing processes or failing to follow proper quality control procedures.
- Distribution and sale: Misbranding can occur if a product is sold in a manner that is misleading or deceptive. This can include selling products that are past their expiration date, selling counterfeit products that are labeled as genuine, or misrepresenting the quality or origin of a product.
Conclusion:
Mislabeling and misbranding have significant consequences, affecting consumer trust and safety. While regulatory bodies like the FDA and FTC enforce standards, it's vital for consumers to stay informed and proactive. Combating these issues requires a collective effort from all parties involved to ensure a fair, transparent, and safe market.
Related links:
- To get a free evaluation of your false advertising case, complete this form.
- To understand whether federal law preempts state law on labeling of food, dietary supplements, or medications, click Does Federal Law "Preempt" (Block) State Lawsuits for Humane-Washing of Meat Labels?
- Learn more about Vitamins & Dietary Supplements.
- For an expansive look into humane washing, click Is Humane-Washing of Meat and Poultry False Advertising?
- Learn about the 4 purposes of advertising, along with numerous common myths in false adverting.
- For a comprehensive review of New York law, click The Ultimate Guide to False-Advertising Law in New York: 22 FAQs.
- Click here to learn more about humane washing, green-washing, and Nutri-washing.
- Were you Misled?: Complete that form for a free legal analysis to determine if you were deceived by humane-washing or green-washing.
- A library of links relating to nutrition, animal welfare, and advertising law.
To speak with Jesse Langel personally about these types of claims, contact him or complete this tailored questionnaire.